Which Dye Is Used in Sds Page Technique
Click to see full answer. The actual separation of the proteins mainly depends on the properties of the gel.
![]()
Sds Page The Bumbling Biochemist
In SDS Page polyacrylamide is used as the solid support for the gel.
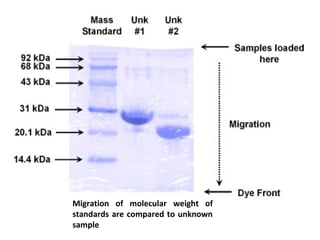
. Electrophoresis Techniques-SDS BN-PAGE In this learning object the learner will be able to Demonstrate Sodium Dodecyl Sulphate SDS-PAGE. The dye used was an acidic mixture of Coomassie Brilliant Blue G-250 purchased from Bio-Rad Laboratories IncHercules CA. The most commonly used form of polyacrylamide gel electrophoresis is the Sodium.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis. In SDS-PAGE the use of sodium dodecyl sulfate SDS also known as sodium lauryl sulfate and polyacrylamide gel largely eliminates the influence of the structure and charge and proteins are separated solely based on polypeptide chain length. It will take approximately one hour to properly stain the SDS-PAGE gel.
Glycerol is added to protein samples before they are loaded to the wells of PAGE. Electrophoresis Multiple Choice Questions and Answers for competitive exams. These stains either use the G-250 colloidal or the R-250 form of the dye.
Both a and b e. 1 2 The procedure involves initial denaturation of component proteins with an anionic detergent that also binds to them imparting to all proteins a negative charge proportional to their. This treatment allows the visualization of proteins as blue bands on a clear background.
An anionic detergent that binds uniformly to polypeptide chains. Technique to separate proteins on the basis of molecular weight. Bromophenol blue is used as a tracking dye in SDS PAGE as well as Agarose gel electrophoresis.
These short solved questions or. Once the electrophoresis is over SDS-PAGE gel will be stained in Coomassie Stain Solution. Components Present in SDS Page.
Alternatively a chemical denaturant may be added to remove this structure and turn the molecule into an unstructured molecule whose mobility depends only on its length because the protein-SDS complexes all have a similar mass-to-charge ratio. Coomassie blue dyes are a family of dyes commonly used to stain proteins in SDS-PAGE gels. As Coomassie stain also binds to SDS-PAGE gel a SDS-PAGE Destain.
The most commonly used technology to obtain high resolution analytical separation of mixtures of proteins is sodium dodecyl sulfate polyacrylamide gel electrophoresis SDS-PAGE. Protein samples loaded on the SDS-PAGE gel will be run in SDS-PAGE Running Buffer. SDS is known as sodium lauryl sulfate sodium dodecyl sulfate.
This procedure is called SDS-PAGE. The most common method of in-gel protein detection is staining with Coomassie dye. This method is called native-PAGE.
The G-250 variant is a pH sensitive dye that is a very dark blue at neutral pH and a much lighter tan color at acidic pH. The system is primarily made with one of these previously mentioned components and gel that is known as a polyacrylamide. Chemical polymerisation of acrylamide gel is used for SDS-PAGE.
Coomassie Brilliant Blue is a typical quick and dirty SDS-PAGE gel stain. It can be initiated by ammonium persulfate and the quaternary amine NNNN- tetramethylethylenediamine TEMED. SDS to denature the polypeptides Dithiothreitol DTT to break disulfide bonds covalent bonds between cysteines Glycerol to make the sample dense enough to sink into the well Bromophenol Blue a negatively charged dye to monitor gel progress.
SDS to assist denaturing and to provide a net negative charge to the protein glycerol to allow the samples to sink into each well bromophenol blue to visualize the lysate and an ionic buffer. STAINING The gel after electrophoresis is put in a solution of Coomassie Brilliant Blue prepared in. Colloidal Coomassie stains can be formulated to effectively stain proteins within 1 hour and requires only water no methanol or acetic acid for destaining.
These short objective type questions with answers are very important for competitive exams like IIT-JEE AIIMS etc. N N N N-tetramethylethylenediamine TEMED. Demonstrate Blue Native BN-PAGE Learning Objective Electrophoresis Techniques-SDS.
-bound SDS contributes a large net negative charge. Vortex each sample and incubate at 95 degrees Celsius for five minutes to completely denature the proteins. Due to the negative charge protein molecules migrate toward the positive charge end of the gel and separates according to their molecular masses.
The function of glycerol is to _____. Once electrophoresis is complete the gel can be stained using colored dyes such as Coomassie Brilliant Blue or ethidium bromide to make the separated proteins appear as distinct colored bands on the gel. Polyacrylamide gel electrophoresis PAGE is a technique widely used in biochemistry forensic chemistry genetics molecular biology and biotechnology to separate biological macromolecules usually proteins or nucleic acids according to their electrophoretic mobility.
As well as Board exams. The electrophoresis apparatus used was a Mini-Protean III Gel Electrophoresis Apparatus purchased. SDS-PAGE is used for.
The gels are soaked in dye and excess stain is then eluted with a solvent destaining. In SDS-PAGE the use of sodium dodecyl sulfate SDS also known as sodium lauryl sulfate and polyacrylamide gel largely eliminates the influence of the structure and charge and proteins are separated solely based on polypeptide chain length. Another technique utilized in this lab is SDS-PAGE or sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Bio-Rad offers Coomassie stains in four formats. Which dye is used in SDS PAGE technique. Which of the following is used as a tracking dye in SDS-PAGE of protein.
The sample buffer used for SDS-PAGE contains a tracking dye bromophenol blue BPB which will migrate with the leading edge of the proteins being separated on the gel. Coomassie Brilliant Blue is the stain used to stain the protein in SDS PAGE. When associated with protein it remains blue even at low pH.
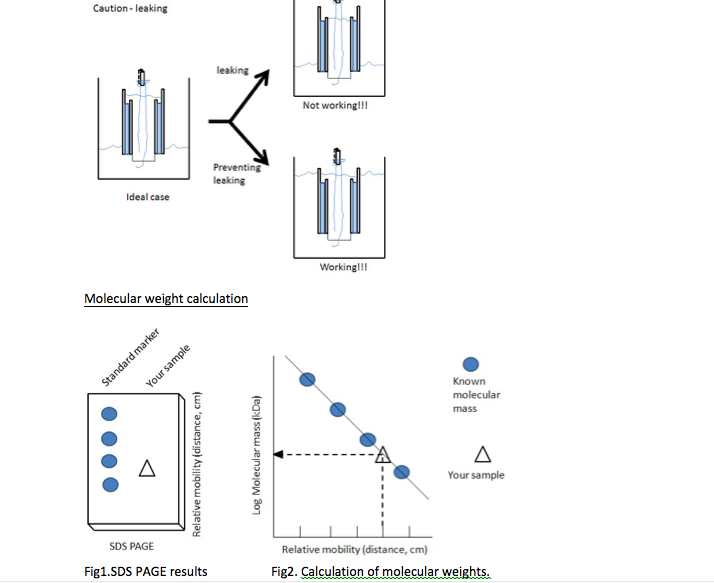
Solved Experiment 8 Sds Page Sodium Dodecyl Sulfate Chegg Com
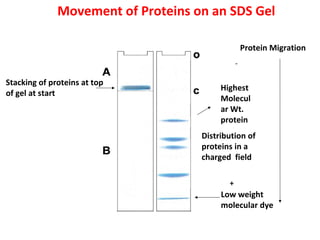

Comments
Post a Comment